Published February 8, 2023 | 3 min read
Key Points
- Recent M&A activity around sickle cell disease companies has highlighted new pharma focus on non-malignant hematologic diseases.
- Gene therapy and gene editing continue to hold out the possibility of a functional cure, though logistics, safety and cost are concerns.
- Despite their classification as rare diseases, effective treatments in the space could create a $6bn market by 2028.
In August 2022, Pfizer acquired Global Blood Therapeutics (GBT) for $5.4 billion, cementing a growing trend for increased investor and M&A interest in rare diseases. Just a month later, Novo Nordisk acquired Forma Therapeutics for $1.1 billion to expand its presence in sickle cell disease and rare blood disorders.
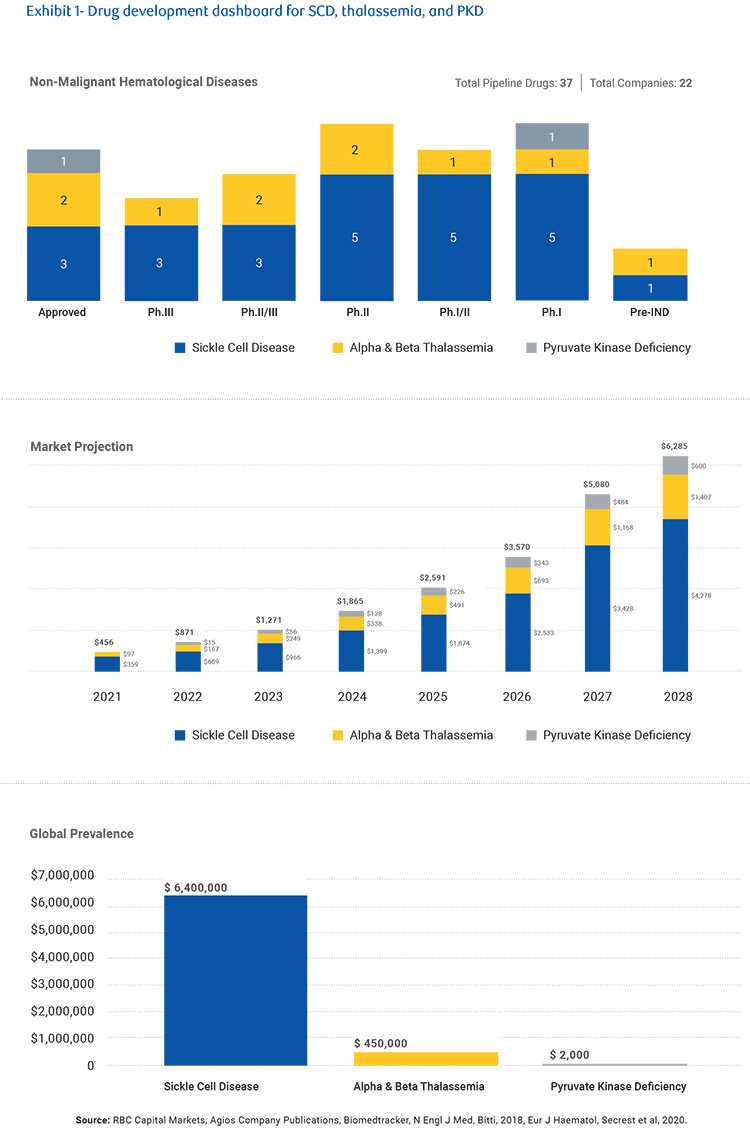
Despite affecting an estimated 100,000 Americans and many more worldwide, sickle cell disease was not widely targeted by drug companies until very recently. The pickup in pharma focus, and the higher investor interest, in non-malignant hematologic diseases like sickle cell, thalassemia, and pyruvate kinase deficiency (PKD), are a result of advances in small molecules and biologics capable of managing these illnesses, and the possibilities for treatment offered by gene and cell therapies.
With multiple upcoming catalysts in the medium-term from key players, momentum is likely to continue in the space, offering the possibility of high-value novel therapies and technologies for treatment, and the eventual creation of one-and-done cures.
The new focus on non-malignant hematologic diseases
Around 300,000 babies every year are born with the genetically-inherited mutation that causes an abnormal, sickle-shaped red blood cell. These sickle cells can impair blood flow, causing painful crises, anemia, chest pain, stroke, kidney disease and death. Up until recently, sickle cell disease symptoms were managed with opioids for pain relief, blood transfusions to manage anemia and avoid stroke, antibiotics and vaccines for infections and spleen removal in cases of spleen enlargement. In cases where siblings were a matched donor, there was also the possibility of hematopoietic stem cell transplantation (HSCT) as a potential cure.
But today treatment includes new drugs that can manage anemia and other blood flow restriction complications (known as vaso-occlusive complications or VOCs), along with research into potential gene and cell therapies. New drugs have also been developed for thalassemia and PKD, novel therapies with improved clinical profiles, while small molecules and biologic therapies of various mechanisms of action are in development.
The promise of a cure
Hematopoietic stem cell transplants (HSCTs) are used to treat hemoglobinopathies such as sickle cell disease and beta thalassemia, and can offer patients a one-time treatment resulting in a functional cure. However, it depends on the patient having an HLA-matched sibling donor, and unfortunately only a minority will have a match – estimates suggest only 18% of sickle cell patients, and only 40% of beta thalassemia patients, have a match.
Since the first successful transplant was performed in 1984, only 1,200 sickle cell patients have been able to try the treatment, partially due to a lack of available donors. But with gene therapy and gene editing, it’s possible to build a treatment based on the patient’s own cells, so they wouldn’t need a donor match. The technology creates a functional copy of the patient’s own hemoglobin to transplant back into them and whether a new gene is added (gene therapy) or the exisiting gene is edited (gene editing), it’s called an ex vivo treatment (denoting that the change is done outside the body). Clinical results so far have been promising in how effective they are, but safety is an area of concern. There is a significant preconditioning regimen that the patient has to undergo to prepare for extraction and for the transplant that can leave them susceptible to complications. There’s also little evidence so far on the long-term effects of gene editing.
The preconditioning regimen is also logistically challenging, requiring multiple hospital appointments and follow-up commitments. And as with all one-and-done treatments, there’s an issue around pricing and reimbursements. One recent ex vivo treatment for beta-thalassemia was recently approved and priced at $2.8m, reflective of the size of the patient population as well as the potential for the treatment to act as a functional cure. Pricing for a functional cure for SCD remains to be determined, but reimbursement in this underserved population may be challenging as a large 30% of patients are reliant on Medicaid.
How current interest builds a $6bn market
Although these illnesses are classed as rare diseases, there is a sizable seven-million-strong world wide population involved and a strong pipeline in treatments could create a market worth up to $6 billion by 2028. However, this forecast is largely dependent on success in sickle cell disease, which makes up around 6.4 million of the patients. There are currently 29 drugs in various stages of clinical development, including ex vivo treatments, representing a significant potential pipeline.
Recent M&A activity around sickle cell disease companies has illustrated the heightened pharma interest in non-malignant hematologic diseases and the potential in multiple small molecules, biologics, and gene therapies. For now, that’s increasing investor interest and corporate development in the space. But in the future, it could lead to a huge market and possibly multiple functional cures.
Gregory Renza, Luca Issi, Brian Abrahams, Yinglu Zhang, Lisa Walter, Leonid Timashev and Joe Kim authored “Non-malignant Hematologic Diseases - Emerging Approaches in Sickle Cell Disease, Thalassemia, and Pyruvate Kinase Deficiency,” published on October 31, 2022. For more information about the full report, please contact your RBC representative.





